Abstract
Background: Clonal hematopoiesis (CH) in known myeloid-related genes has been linked to chronic inflammation and immune dysregulation with aging. Although causal directionality is still uncertain, CH in myeloid cells has been hypothesized as etiologic of inflammation, by a mechanism of altered, pro-inflammatory terminal myeloid effectors. Here, we modeled the contribution of age versus systemic inflammation for CH incidence in a spectrum of vasculitis syndromes occurring in young, middle aged, and older patients.
Methods: Patients with giant cell arteritis (GCA, n=63; median age [range]= 72 [45-88]), ANCA-associated vasculitis (AAV, n=47; median age [range]= 60 [19-77]), and Takayasu's arteritis (TAK, n=70; median age [range]= 33 [3-71]) were screened for CH in peripheral blood with error-corrected targeted sequencing (minimum variant allele fraction [VAF] of 0.5%). Genomic results were compared to age-matched healthy controls (HC, n=151) and correlated with clinical variables. Logistic regression was used to assess associations of age and vasculitis with CH; relative contribution of predictor variables was assessed using standardized beta coefficients. Clonal hierarchies in GCA were assessed by proteogenomic single-cell DNA (scDNA; Mission Bio).
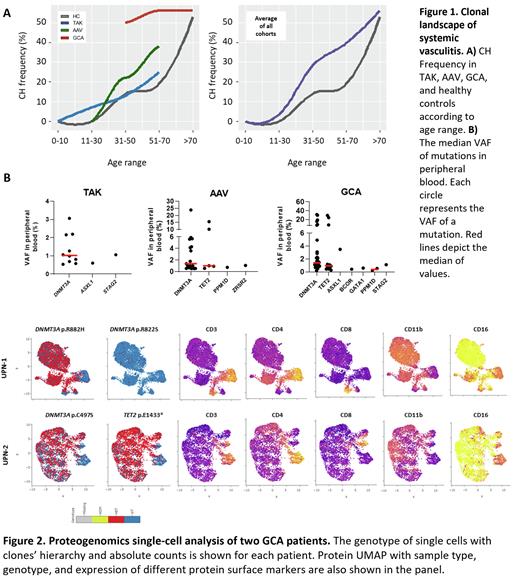
Results: CH incidence in GCA, AAV, and TAK was 59% (37/63), 32% (15/47), and 13.0% (9/61), respectively; except for TAK, these frequencies were substantially higher than age-matched HC (Figure 1). CH was frequently at low median VAFs (<1.5%) and most patients had only one mutation, regardless of the type of vasculitis (median of 63% vs 37% with >2 mutations). In all cohorts, DNMT3A was the most commonly mutated gene, found in 44%, 23%, and 7% of GCA, AVV and TAK patients, respectively (Figure 1). TET2 mutations were absent in the younger TAK patients but the frequency increased in older patients with GCA (13%) and AAV (5%). Clone size and the number of patients with both DNMT3A and TET2 mutations increased with aging in association with AAV and GCA. In serial samples, clones were stable over time in most patients, regardless of their clinical status. Using logistic regression modeling, age and vasculitis were independently associated with CH (Age: B=0.05, standardized b=0.96, p<0.0001; Vasculitis: B=0.53, standardized b=0.46, p=0.004).
Vasculitis patients with CH had lower percentages of lymphocytes (18% vs 25%, p=0.03) and higher absolute neutrophil counts (6.0 vs 5.1 K/uL, p=0.04) in peripheral blood than those without CH. Adjusting for daily prednisone dose, the neutrophil-to-lymphocyte ratio was significantly associated with the presence of CH (B=0.07, p=0.02). Among patients with mutations, clone size was positively associated with monocyte count (B=0.83, p=0.01). In two GCA patients with both DNMT3A/TET2 mutations, scDNA sequencing showed driver clones were mutated in DNMT3A and later acquired a TET2 mutation (Figure 2). UPN-1 had 4 independent clones: a clone with a DNMT3A p.R882H that acquired two different TET2 mutations in independent subclones, and a different DNMT3A p.R882S subclone. As expected, myeloid cells (neutrophils, monocytes, dendritic cells) and B cells were mutated and showed markers of cell activation. T CD3+CD4+ or CD8+ lymphocytes were mostly wild-type, but in both patients 20-30% of T cells were mutated and remarkably expressed myeloid markers linked to cytotoxic activity (CD3+/CD4+orCD8+/CD11b+/CD16+). When simulated in vitro, monocytes from patients with DNMT3A or TET2 mutations vs. wild-type had increased CCL2/ MIP-1a/ MIP-1b but not TNF-α secretion. TNF-α and IL-1RA secretion was significantly lower when patients had both DNMT3A/TET2 mutations. CH mutations were detected in inflamed temporal arteries in patients with GCA at lower VAFs than in peripheral blood (<0.5% vs. 1-2%, respectively).
Conclusion: In vasculitis, age and inflammation were independent contributors for CH, with aging much more strongly associated. DNMT3A mutated cells dominated the clonal landscape. CH was associated with skewing of blood counts towards myeloid lineages, most of which were mutated and of activated phenotype. Although mutated cells may contribute to propagation of inflammation, the clonal selection appears to be consequence of the inflammatory environment rather than directly initiating systemic vasculitis.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal